
Business Development and Innovation
Licensing in & out and partnering strategy
Licensing-in
Laboratorios Gebro Pharma Licensing-In: local player of choice for regional deals
The business model of Gebro is based on building up strong brands through active and high-quality promotion to specialists, with a significant investment in scientific activities and value-added marketing. Gebro, proven to be the best marketing partner in its class, focuses on the acquisition, licensing, and development of specialized and innovative healthcare products for human health. Committed to improving the quality of life for individuals, Gebro actively seeks prescription products in the following areas:
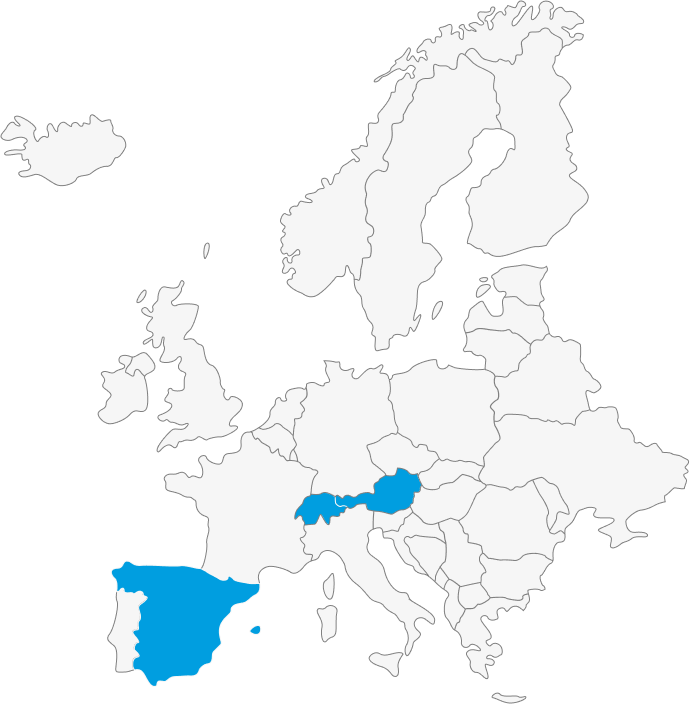

Other European/EEA markets (long-term network partners)
Gebro Group
We are a privately-owned family company focused on partnering with a brand-oriented approach. On an international scale, we offer:
- Own products available for licensing.
- Own structures for products licensed in the established markets of the Gebro Group (Austria, Spain, and Switzerland), as well as for markets within our partner network.
Discover more in:
https://gebro.com
Our value proposition is based on these four pillars:
Partnership oriented and agile in deal making
Full commercial sales and marketing capabilities
Strong know-how in regulatory affairs
Commitment with all our products
Licensing-out
The Gebro Holding Group markets its own brands and also enters into agreements with third parties for their marketing.
We put healthcare professionals at the center of its momentum. We are passionate about creating our own innovation projects by partnering with recognised public and private research groups and with national institutions of the highest level.
In our headquarters in Austria, we offer full development services – from the idea to the finished product.
- A galenical laboratory equipped with state of the art technology.
- Development, scale-up and troubleshooting of solid, liquid and semi-solid dosage forms.
- Recipe development including production of pre-stability samples.
- Scale-up and support of the product transfer to our production facilities through to process validation.
Development and validation of all common analytical methods.;
Chemical-physical analysis of test series, pre-stability and ICH stability samples.
Discover more in:
https://gebro.at/Innovation model
First we listen, and then we innovate
At Gebro Pharma, innovation drives us to rethink, reinvent, and optimize medications, placing patients and healthcare professionals at the core of this unstoppable movement. We listen to their needs, and then we innovate.
Our innovation model is based on value-added drugs. We employ the VAM (Value Added Medicines) model, grounded in the following principles:
Patient Focus
Rethinking
Reinventing
Optimizing medications
Improving market access
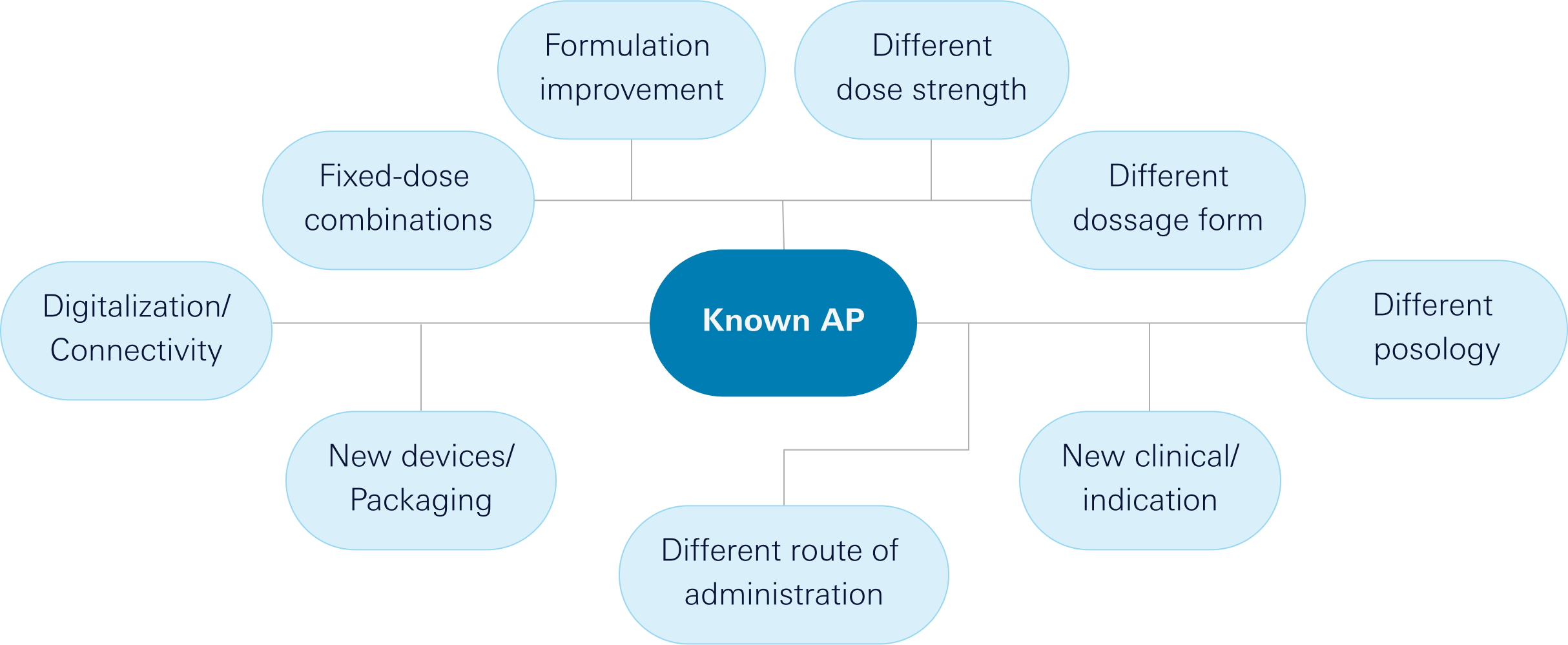
The added value we provide in medications is particularly evident in these aspects:
Reformulation
Clinical Program
Technology
Our innovation model focuses on the following elements:
Value Proposition
From our Innovation Department, we generate ideas for drugs based on known molecules but providing an additional health benefit to patients and their caregivers. This added benefit to medicines can be in terms of efficacy, safety, dosage, administration route, formulation, or combination, among others. We are passionate about creating our own innovation projects by collaborating with renowned public and private research groups and institutions at the highest level within the territory. We primarily focus on areas where we excel: autoimmune diseases, pain, respiratory and uroginecology.
Our strength in making agreements is based on these points:
Agility in decision-making and agreement negotiations
Know-how in Regulatory Affairs
Strong relationships with authorities for price and reimbursement negotiations
Availability of internal resources for promotion and investment in new products
Long-term commitment to all our products
Development of commercial activities with added value
We have a virtual Research and Development model in which the organization works with a limited number of internal staff and utilizes external resources, technologies, and facilities on demand to develop its R&D projects. This model offers numerous advantages, such as reduced capital requirements, overall costs, and financial risks, limited infrastructure costs, and, of course, greater flexibility.
Market pull:
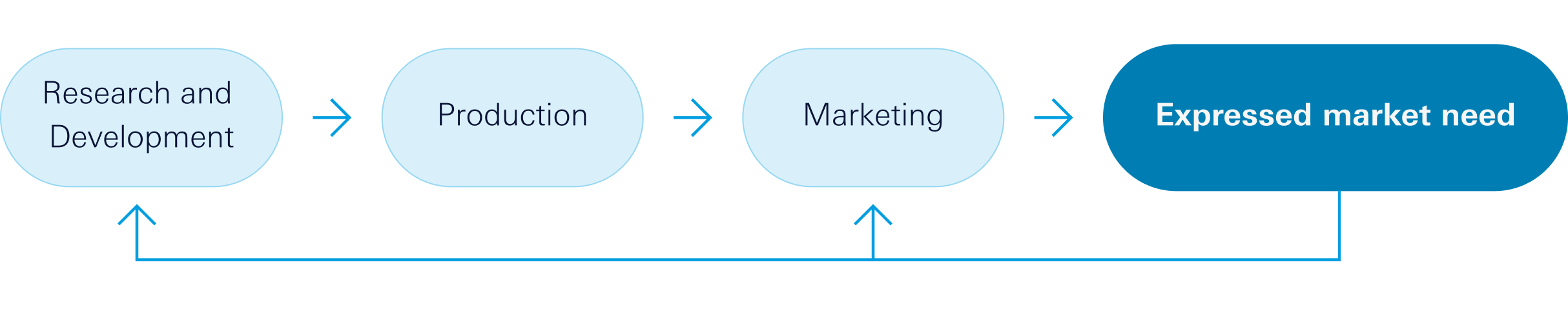
Pipeline LGP Q32023
Our pipeline for 2023 focuses on these therapeutic areas.